
When you hear the word ‘argon’, it doesn’t spring to mind as quickly as hydrogen or helium.
The nature is also moderately difficult to remember…
However, knowing what it is used for in society and why argon is used can help you remember its properties. Let’s look at this element together, as it is widely used in ordinary households and in the medical field, and is surprisingly familiar to us!
Contents
Properties and characteristics of Argon

It has the property of being stable and not causing chemical reactions. 1% in air and is cheaper to obtain than other inert gases. This element is mainly used in laboratories, such as universities.
How Argon is used in society
Argon is used as a component of ‘medical lasers’, ‘sputtering equipment’ and ‘fluorescent lamp contents’.
Let’s look at each of them.
Argon is useful for “Medical laser (retinal treatment)”
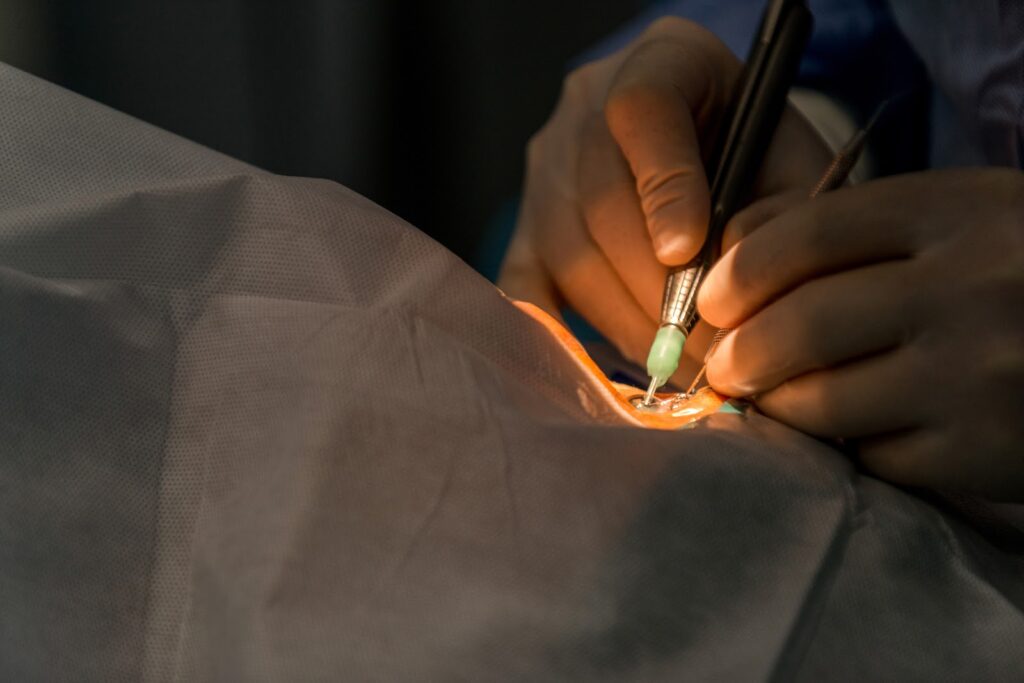
This laser is often used in ophthalmology and is performed as a surgical procedure to reduce the progression of retinal disease. Various elements are used in medical lasers, but the ‘argon laser’, which uses argon ions, is used in the treatment of retina.
The argon laser is used by many ophthalmologists because it is less easily absorbed by water and more easily absorbed by red (blood), resulting in less strain on the eye and faster recovery.
Argon is useful for “sputtering equipment”

Argon is also used in sputtering equipment for film deposition.
This makes use of the fact that argon is stable and does not cause chemical reactions. The use of stable argon makes it possible to deposit copper as copper and iron as iron. (There is also a technique called reactive sputtering, in which argon is mixed with a little oxygen or nitrogen for sputter deposition.)
Here are some examples of sputtering equipment.
List of sputtering equipmentArgon is useful “contents of fluorescent lamps”

Two types of gas are enclosed in fluorescent lamps: mercury gas and argon gas. Argon gas is mainly put in to keep the discharge constant.
Incidentally, let’s talk about why fluorescent bulbs glow.
The flow of electricity causes collisions between electrons and mercury atoms, which emit ultraviolet radiation. This ultraviolet radiation hits the phosphor coated on the surface of the fluorescent lamp, which illuminates it brightly.
History of Argon

Chemical symbol: Ar
English Name: Argon
The word argon is derived from the Greek word ‘aergon’, meaning ‘that which does not work’. As for the discovery, Ramsey and Reilly found it in the air in 1984.
Summary
In this article, we have explained ‘ Argon ‘.
The key points of this article are as follows.
- Argon does not cause chemical reactions and has stable properties
- ‘Used in medical lasers’, ‘sputtering equipment’, ‘fluorescent lamp contents’, etc.
- The etymology of argon is ‘that which does not work’.
The other elements studied in high school are summarized in the following articles. Please see also the following pages for a further understanding!






